Choose Your Language
Request a Quote
Plastic Solutions for Healthcare
- Home
- Industries
- Healthcare
In the ever-changing healthcare market from at-home medical devices to antimicrobial properties and medical grade materials, we work with you to help meet strict industry regulations. Many of our medical grade thermoplastics can withstand various sterilization methods, including autoclave, gamma, e-beam and EtO. Some materials are potentially biocompatible and adhere to healthcare management change policies.
We work with you to help comply with the following regulations:
We also offer medical grade plastics that are:
Plastics have been used widely to create medical tools and devices like surgical gloves, syringes, prosthetics, insulin pens, IV tubes, catheters, inflatable splints, etc. Many applications that use plastics are designed to help prevent the spread of disease and germs.
Many medical device housings are made from ABS, PC/ABS and PC/PET. Medical thermoplastic elastomers (TPE) and PMMA materials are also very common plastics used in medical device applications, including fluid and drug delivery, healthcare packaging, and cardiovascular and blood care.
Medical grade plastics typically have properties that meet certain regulatory guidelines and characteristics, including chemical resistance, durability, antimicrobial properties, flame retardancy, clarity, impact resistance, indoor UV stability, and colorability.
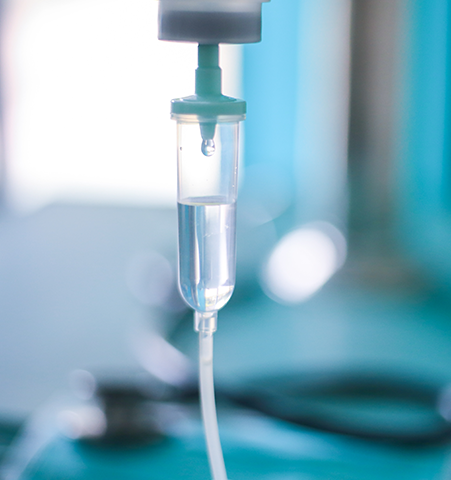
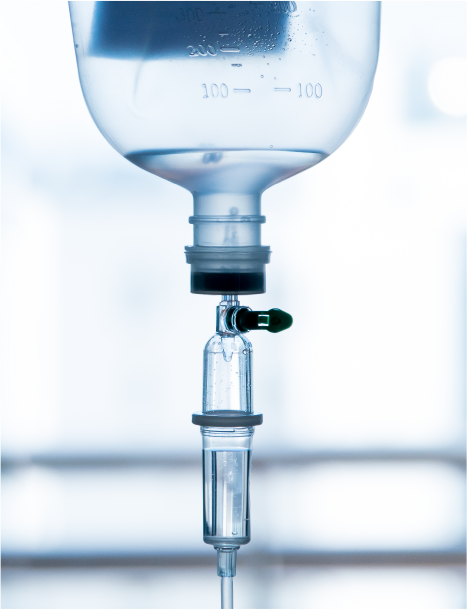
These applications require a high level of sterility, biocompatibility, chemical resistance, clarity, as well as low extractables for auto-injectors, inhalers, syringes, catheters, tubing, lures and drip chambers.
Many different plastics may be used in fluid and drug delivery applications, including
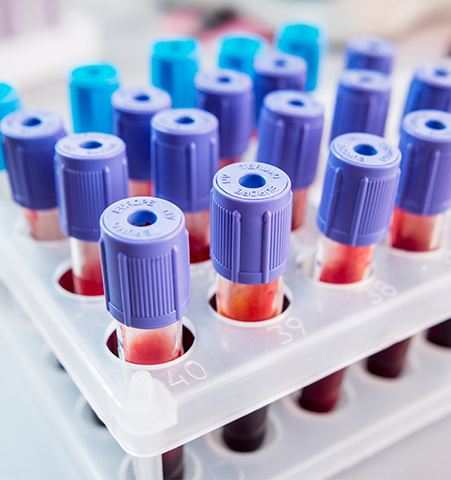
For extra-corporeal systems, blood collection, separation and filtration, and storage applications, we can help you identify material options that offer biocompatibility, chemical resistance, clarity, sterility, and good flow for processing.
Certifications required for plastics used in blood care applications may include pre-market notification (510K), pre-market approvals (PMA), CFR21 device classification, ISO-10993 & US Pharmacopeia Class VI, FDA device Classification (Class - I, II) approvals, and Master Drug File (DMF).
PC, PC alloys, PMMA, and PS are often the most common types of plastics used in cardiovascular and blood care applications.

Our healthcare thermoplastics can help protect packaged products from moisture, light, oxygen, and contaminants. Primary and secondary applications include packaging for pharmaceuticals and medical devices – such as, closures, pouches, and prefilled syringes. Sustainable packaging solutions are also available to help you meet your sustainability targets.
PS, PE, PP, and TPEs are commonly found in pharmaceutical packaging.
Plastics are often used in pharmaceutical packaging due to sterilizability, sealing and barrier properties, clarity, and cost effectiveness.

Applications that can benefit from using plastics include petri dishes, pipettes, cryogenic storage tubes, droppers, and specimen analysis equipment.
Thermoplastics can deliver required properties for these applications, including low-temperature resistance, low-protein binding, clarity, rigidity, and sterility.
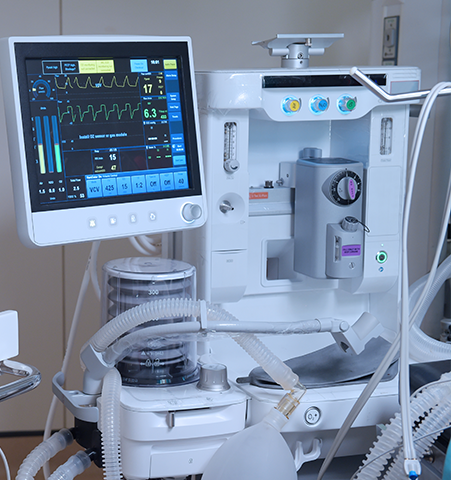
Monitoring and imaging devices comprise a diverse range of applications from hand-held and small devices, such as pulse oximeters and blood pressure monitors, to larger transportable devices, like ultrasound and anesthesia delivery, to very large stationary equipment, including X-ray, CT, MRI, and PET imaging machines.
PC, PC/ABS, PC PBT, ABS are all types of plastics that can be found in monitoring devices and imaging healthcare applications. Our experts can help you identify thermoplastics that provide a variety of required properties, including chemical resistance, durability, flame retardancy, impact resistance, indoor UV stability, and colorability, to meet your application needs.

We offer materials that deliver colorability, sterility, strength, and weight for non-woven disposable applications, including diapers, incontinence products, surgical disposal gowns (PPE), face masks, and feminine hygiene products.
Flexibility, costs, and disposability are often the main benefits of using plastic in non-woven disposables.
The most commonly used plastics for non-woven disposables are Polyethylene (PE), polypropylene (PP), and polyethylene terephthalate (PET).

Bone cement mixers, external bone fixation devices, instrument handles, trays and cases require thermoplastics that deliver biocompatibility, sterility, dimensional stability, impact, and chemical resistance and colorability.
Strength, toughness and stiffness, as well as skin contact, are excellent characteristics of orthopedic plastics.
Nylon, glass filled nylon, PC/PBT, PC/ABS, and TPUs can often be excellent choices for orthopedic plastic devices.

Our plastics solutions for respiratory therapy applications offer characteristics such as soft touch, comfort, and durability. Common applications include respiratory masks, ventilators, CPAP devices, nebulizers, and tracheal tubes.
Plastics are used to help prevent environmental stress cracking and deliver on required properties, including, clarity, toughness, flexibility and stiffness, bondable, chemical resistance, breathable, antimicrobial properties, no or low migrating leachable emitting, overall cost effectiveness, and recyclability.
Common plastics for respiratory therapy applications include clear PVC, PMMA, PS, and TPEs.

As surgical devices, including trocars, retractors, speculums, forceps, and electrosurgical instruments, become smaller and more complex, we offer plastics material solutions that can provide strength, durability, and design freedom for miniaturized instruments.
Common certifications for plastics that are used in surgical instruments may include pre-market notification (510K), pre-market approvals (PMA), CFR21 device classification, ISO-10993 & US Pharmacopeia Class VI, FDA device Classification (Class - I, II) approvals, and Master Drug File (DMF).
Properties of plastics used in surgical devices often include chemical resistance, lipid resistance, sterilizability, toughness, impact resistance, clarity, and USP class VI approvals.